Abstract
Introduction Treatment with Hypometilating Agents (HMA) of unfit patients (pts) with Acute Myelogenous Leukemia (AML) and High-Risk Myelodysplastic Syndromes (HR-MDS) is often difficult in the standard Day-Hospital (DH) setting, due to the number of hospital admissions required and the frail clinical conditions of pts. In the Viterbo province, accounting for 3612 Km 2 divided into 60 municipalities, is operative an Unit of Domiciliary Hematologic Care (UDHC) for clinical assistance to frail pts with hemopathies.
Aims To evaluate the role of the UDHC compared to standard DH setting in the active frontline treatment with HMA +/- venetoclax (VTX) of pts with AML/HR-MDS.
Methods All pts with newly diagnosed AML/HR-MDS unfit for intensive care and treated frontline with HMA from 1/2010 to 4/2021 were analysed.
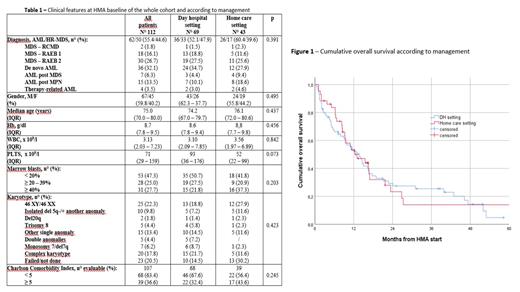
Results In this study period, 112 pts (62 AML/50 HR-MDS) received HMA (azacytidine in 105 cases and decitabine in 7 cases): six pts added VTX to HMA. As concern therapy management, 69 pts (61.6%) were treated in a standard DH setting and 43 (38.4%) were followed by UDHC: pts were allocated to DH or home care setting by responsible physician based on clinical conditions, comorbidities, caregiver availability and distance from hospital. The main features at baseline of HMA in the whole cohort and according to management are reported in the Table 1. Median interval from diagnosis to HMA initiation was 0.9 months (IQR 0.5 - 3.0). Median number of HMA cycles administered was 9 (IQR 4 - 16). The overall response rate (ORR), including complete response, partial response and hematologic improvement, was 43.7% (48/112 pts) in the whole cohort, without differences according to management [29/69 (42.0%) in DH vs 19/43 (44.1%) in home care, p=0.797]. Infections were also equally reported [46/69 pts (66.6%) in DH vs 31/43 (72.0%) in home care setting had at least 1 infection, p=0.362]. Median response duration of the whole cohort was 10.0 months (95%CI 5.7 - 14.2), without differences according to management [8.7 months (95%CI 7.0 - 10.3) in DH vs 13.0 months (95%CI 8.3 - 17.6) in home care, p=0.460]. Median Overall Survival (OS) of the whole cohort was 13.0 months (95%CI 9.7 - 16.2): median OS of pts treated in DH was 13.7 months (95%CI 9.9 - 17.4) compared to 13.0 months (95%CI 6.7 - 19.3) of pts managed by UDHC (p=0.753) (Figure 1).
Conclusions Home care management of HMA for unfit AML/HR-MDS pts is feasible and effective, with results similar to those achievable in a standard DH setting: this approach is thus adequate to offer active therapies in a fraction of frail pts with AML/HR-MDS considered up to now ineligible.
Latagliata: Novartis: Honoraria; Pfizer: Honoraria; BMS Cellgene: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal